Class: Advanced Chemistry: Organic Chemistry
Grade: 12
Teacher: Alison Livingston
Students in Organic Chemistry participated in a lab that challenged them to investigate the process of oxidation-reduction reactions. The goal of the lab was to determine the amount of Vitamin C present in various liquids using the process of titration. By utilizing vitamin C and the metabolization of the human body as a model system, students gained a deeper understanding of how oxidation occurs within their bodies and the world around them.
The process started with each group answering pre-lab questions and narrowing down a hypothesis. Students predicted which liquids would have the most Vitamin C present and analyzed why Vitamin C levels may differ between fresh, frozen, and canned orange juice. After hypothesizing, each group got to work testing a wide range of juices including fresh orange juice, Motts Apple Juice, Simply Orange Juice, Martinellis Apple Juice, and milk.

Organic chemistry is one of those classes that people might think is intimidating, so I try to bring in real-world examples.
-Alison Livingston, Science Department Head
Students introduced an oxidizing agent drop by drop into the liquids they were testing. A color change within the liquid would signal oxidation, prompting groups to record how many drops it took to reach that point. Using this data and a series of calculations, students were able to determine the vitamin C contents within each juice.
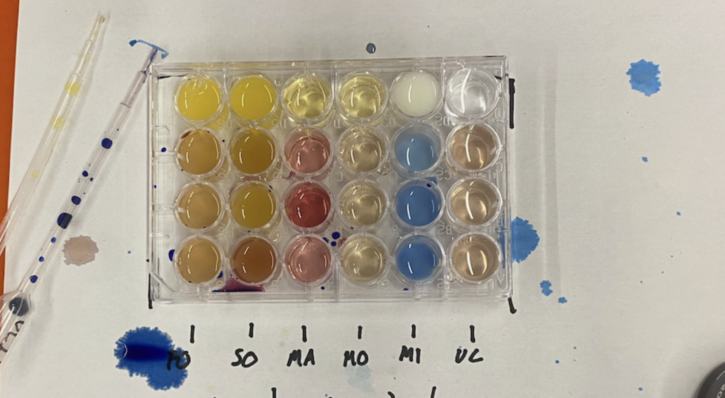
Before reaching conclusions, students answered a series of post-lab questions that tasked them with completing calculations, analyzing their findings, and doing additional research into the topic. Students explained each step of their work and included calculations within their final reports. During their analyses, many students were interested in the differences between the vitamin C levels advertised on nutritional labels and the actual vitamin C contents within each juice.
One thing that surprised me was the disparity between the advertised levels of vitamin C and the experimental value of vitamin C. The calculated values were very different from the nutritional labels, and I was surprised that some companies under-advertise how much vitamin C they are putting into their juice.
-Student reflection
Not only did the lab illuminate how vitamin C functions within common juices, it also contextualized oxidation-reduction reactions for students in an accessible way.
The results of this experiment demonstrate the instability of vitamin C when exposed to various conditions. The results demonstrate that vitamin C oxidizes easily when exposed to the air, which occurs most during processing.
-Student reflection

